Archive for category powder_tapping
sizing lactose 02
Posted by () in Open Notebook Science, powder_tapping on May 19, 2013
I went ahead and developed my own script to look at the 10th, 50th, and 90th percentiles for the particle sizing done on the SympaTEC HELOS laser diffractor device. As I suspected, the machine does in fact plot the cumulative distribution of particles and fits the data to a Sigmoid. It then calculates the 10th, 50th, and 90th percentiles and reports those values back to a txt file. The script is below the summary table. I will note that I got a NAN for one of the calculated measurements which, caused me to toss out that data point for my calculations. For completeness, I also removed it from the reported values.
Forgive the markdown table below, wordpress doesn’t recognize how to deal with markdown for some reason. It also doesn’t understand what WHITESPACE is supposed to mean. Nonetheless, it is clear that the machine is doing what one would expect as my calculated values are within error of the values reported. This is good as it is no longer a blackbox to me and I will report the values that it gives.
Pharmatose 150M | Percentile | Reported Average | Reported STD | Calculated Ave | Calculated STD | |:----------:|:----------------:|:------------:|:--------------:|:--------------:| | 10th | 121.9 µm | 6.6 µm | 117.1 µm | 6.5 µm | | 50th | 190.7 µm | 11.4 µm | 191.9 µm | 11.8 µm | | 90th | 268.2 µm | 22.6 µm | 260.0 µm | 20.8 µm | SuperTab 11SD | Percentile | Reported Average | Reported STD | Calculated Ave | Calculated STD | |:----------:|:----------------:|:------------:|:--------------:|:--------------:| | 10th | 119.7 µm | 7.9 µm | 116.3 µm | 6.1 µm | | 50th | 187.9 µm | 15.1 µm | 189.1 µm | 16.6 µm | | 90th | 271.4 µm | 39.9 µm | 262.4 µm | 32.9 µm | SuperTab 30GR | Percentile | Reported Average | Reported STD | Calculated Ave | Calculated STD | |:----------:|:----------------:|:------------:|:--------------:|:--------------:| | 10th | 117.9 µm | 26.5 µm | 117.9 µm | 18.1 µm | | 50th | 183.5 µm | 21.0 µm | 189.9 µm | 13.4 µm | | 90th | 257.4 µm | 28.3 µm | 256.4 µm | 19.2 µm |
# Import statements. import numpy as np from scipy.optimize import curve_fit # Input data. size = [ 4.50, 5.50, 6.50, 7.50, 9.00, 11.00, 13.00, 15.50, 18.50, 21.50, 25.00, 30.00, 37.50, 45.00, 52.50, 62.50, 75.00, 90.00, 105.00, 125.00, 150.00, 180.00, 215.00, 255.00, 305.00, 365.00, 435.00, 515.00, 615.00, 735.00, 875.00 ] # Pharmatose 150M pharmatose_150M_od43_63 = [ 2.16, 2.38, 2.54, 2.67, 2.82, 2.96, 3.07, 3.17, 3.28, 3.37, 3.46, 3.59, 3.77, 3.94, 4.10, 4.31, 4.67, 5.43, 6.96, 11.20, 21.73, 41.64, 67.86, 89.30, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] pharmatose_150M_od08_90 = [ 1.62, 1.87, 2.08, 2.26, 2.49, 2.73, 2.91, 3.08, 3.20, 3.27, 3.27, 3.27, 3.27, 3.27, 3.35, 3.52, 3.80, 4.40, 5.81, 10.75, 25.32, 51.46, 81.69, 97.52, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] pharmatose_150M_od15_83 = [ 2.27, 2.49, 2.63, 2.74, 2.84, 2.93, 2.93, 2.93, 2.93, 2.93, 2.93, 2.93, 2.93, 2.93, 3.01, 3.13, 3.36, 3.92, 5.31, 9.84, 21.84, 42.60, 67.85, 88.46, 99.02, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] pharmatose_150M_od15_52 = [ 1.69, 1.90, 2.06, 2.18, 2.33, 2.47, 2.57, 2.57, 2.57, 2.57, 2.57, 2.57, 2.57, 2.57, 2.68, 2.81, 2.98, 3.38, 4.42, 8.12, 18.74, 38.48, 63.38, 84.92, 94.39, 96.65, 100.00, 100.00, 100.00, 100.00, 100.00 ] pharmatose_150M_od09_31 = [ 2.25, 2.54, 2.77, 2.96, 3.17, 3.38, 3.53, 3.66, 3.77, 3.84, 3.84, 3.84, 3.84, 3.92, 4.05, 4.25, 4.55, 5.26, 7.09, 13.46, 29.42, 53.34, 79.60, 94.53, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] pharmatose_150M_od21_24 = [ 2.29, 2.53, 2.70, 2.82, 2.94, 3.03, 3.03, 3.03, 3.03, 3.03, 3.03, 3.09, 3.23, 3.38, 3.53, 3.73, 4.07, 4.78, 6.38, 11.15, 22.47, 40.47, 61.63, 80.36, 94.20, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] pharmatose_150M_od13_58 = [ 2.42, 2.66, 2.82, 2.93, 3.05, 3.14, 3.14, 3.14, 3.14, 3.14, 3.14, 3.14, 3.14, 3.14, 3.22, 3.36, 3.60, 4.11, 5.23, 8.67, 18.17, 36.99, 62.47, 83.56, 94.95, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] pharmatose_150M_od07_26 = [ 3.11, 3.51, 3.81, 4.03, 4.27, 4.48, 4.60, 4.70, 4.70, 4.70, 4.70, 4.76, 4.87, 5.04, 5.25, 5.57, 6.03, 6.97, 9.19, 16.09, 32.51, 57.07, 81.43, 94.38, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] pharmatose_150M_od15_40 = [ 2.57, 2.90, 3.15, 3.33, 3.51, 3.66, 3.75, 3.75, 3.75, 3.75, 3.75, 3.80, 3.92, 4.06, 4.19, 4.36, 4.59, 5.09, 6.23, 9.78, 19.14, 36.31, 58.95, 82.39, 98.64, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] pharmatose_150M_od16_94 = [ 2.60, 2.89, 3.09, 3.22, 3.35, 3.44, 3.44, 3.44, 3.44, 3.44, 3.44, 3.53, 3.66, 3.77, 3.87, 4.02, 4.26, 4.73, 5.79, 9.07, 17.62, 33.27, 53.49, 73.69, 89.55, 97.91, 100.00, 100.00, 100.00, 100.00, 100.00 ] pharmatose_150M_ave = [np.average(item) for item in zip(pharmatose_150M_od43_63, pharmatose_150M_od08_90, pharmatose_150M_od15_83, pharmatose_150M_od15_52, pharmatose_150M_od09_31, pharmatose_150M_od21_24, pharmatose_150M_od13_58, pharmatose_150M_od07_26, pharmatose_150M_od15_40, pharmatose_150M_od16_94)] pharmatose_150M_std = [np.std(item) for item in zip(pharmatose_150M_od43_63, pharmatose_150M_od08_90, pharmatose_150M_od15_83, pharmatose_150M_od15_52, pharmatose_150M_od09_31, pharmatose_150M_od21_24, pharmatose_150M_od13_58, pharmatose_150M_od07_26, pharmatose_150M_od15_40, pharmatose_150M_od16_94)] pharmatose_150M = [ pharmatose_150M_od43_63, pharmatose_150M_od08_90, pharmatose_150M_od15_83, pharmatose_150M_od15_52, pharmatose_150M_od09_31, pharmatose_150M_od21_24, pharmatose_150M_od13_58, pharmatose_150M_od07_26, pharmatose_150M_od15_40, pharmatose_150M_od16_94 ] # SuperTab 11SD supertab_11SD_od20_93 = [ 0.43, 0.50, 0.57, 0.63, 0.71, 0.81, 0.90, 0.99, 1.10, 1.19, 1.29, 1.43, 1.65, 1.88, 2.13, 2.49, 3.07, 4.27, 6.70, 13.32, 29.17, 56.35, 84.37, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_11SD_od12_18 = [ 0.55, 0.61, 0.61, 0.61, 0.61, 0.61, 0.61, 0.61, 0.61, 0.61, 0.68, 0.80, 0.99, 1.21, 1.45, 1.81, 2.36, 3.43, 5.67, 12.27, 28.07, 53.28, 81.44, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_11SD_od08_01 = [ 0.00, 0.07, 0.07, 0.07, 0.07, 0.07, 0.07, 0.07, 0.07, 0.07, 0.16, 0.30, 0.54, 0.83, 1.15, 1.63, 2.41, 3.92, 6.94, 15.44, 33.55, 58.19, 83.75, 97.68, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_11SD_od06_29 = [ 0.00, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.15, 0.31, 0.56, 0.83, 1.12, 1.53, 2.09, 2.92, 4.35, 8.68, 20.48, 40.37, 63.77, 85.46, 99.23, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_11SD_od15_59 = [ 0.00, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.16, 0.32, 0.59, 0.88, 1.18, 1.59, 2.17, 3.14, 4.93, 9.76, 21.00, 38.97, 60.11, 79.39, 94.27, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_11SD_od10_27 = [ 0.00, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.06, 0.14, 0.27, 0.49, 0.74, 0.98, 1.29, 1.69, 2.39, 3.84, 8.10, 18.11, 34.17, 53.57, 71.78, 85.51, 94.19, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_11SD_od09_51 = [ 0.61, 0.76, 0.90, 1.04, 1.24, 1.51, 1.75, 2.03, 2.32, 2.57, 2.85, 3.25, 3.86, 4.46, 4.99, 5.60, 6.31, 7.52, 10.15, 17.87, 34.59, 58.89, 83.86, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_11SD_od07_94 = [ 0.52, 0.60, 0.60, 0.60, 0.60, 0.60, 0.60, 0.70, 0.81, 0.92, 1.04, 1.21, 1.50, 1.81, 2.14, 2.61, 3.26, 4.33, 6.32, 11.81, 24.04, 42.31, 63.21, 81.04, 92.89, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_11SD_od08_40 = [ 0.51, 0.59, 0.59, 0.59, 0.59, 0.59, 0.59, 0.59, 0.59, 0.69, 0.82, 1.01, 1.32, 1.63, 1.93, 2.31, 2.80, 3.64, 5.22, 9.51, 19.31, 34.87, 53.46, 71.73, 86.80, 96.02, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_11SD_ave = [np.average(item) for item in zip(supertab_11SD_od20_93, supertab_11SD_od12_18, supertab_11SD_od08_01, supertab_11SD_od06_29, supertab_11SD_od15_59, supertab_11SD_od10_27, supertab_11SD_od09_51, supertab_11SD_od07_94, supertab_11SD_od08_40) ] supertab_11sd_std = [np.std(item) for item in zip(supertab_11SD_od20_93, supertab_11SD_od12_18, supertab_11SD_od08_01, supertab_11SD_od06_29, supertab_11SD_od15_59, supertab_11SD_od10_27, supertab_11SD_od09_51, supertab_11SD_od07_94, supertab_11SD_od08_40)] supertab_11SD = [ supertab_11SD_od20_93, supertab_11SD_od12_18, supertab_11SD_od08_01, supertab_11SD_od06_29, supertab_11SD_od15_59, supertab_11SD_od10_27, supertab_11SD_od09_51, supertab_11SD_od07_94, supertab_11SD_od08_40 ] # SuperTab 30GR supertab_30GR_od05_40 = [ 0.89, 0.96, 0.96, 0.96, 0.96, 0.96, 0.96, 0.96, 0.96, 0.96, 0.96, 0.96, 1.03, 1.14, 1.26, 1.43, 1.63, 1.90, 2.53, 4.99, 13.15, 30.51, 55.09, 80.77, 98.84, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_30GR_od14_59 = [ 0.82, 0.91, 0.91, 0.91, 0.91, 0.91, 0.91, 0.91, 0.91, 0.91, 0.99, 1.11, 1.29, 1.47, 1.64, 1.88, 2.23, 2.82, 3.96, 7.42, 17.30, 37.26, 64.08, 86.29, 97.81, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_30GR_od14_16 = [ 0.81, 0.90, 0.90, 0.90, 0.90, 0.90, 0.90, 0.90, 0.90, 0.90, 0.97, 1.07, 1.23, 1.40, 1.57, 1.83, 2.19, 2.83, 4.08, 8.10, 19.27, 38.78, 62.03, 80.56, 93.90, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_30GR_od12_68 = [ 1.11, 1.23, 1.32, 1.32, 1.32, 1.32, 1.32, 1.32, 1.32, 1.32, 1.41, 1.55, 1.76, 2.01, 2.29, 2.71, 3.30, 4.15, 5.64, 10.08, 22.32, 43.58, 68.81, 86.73, 96.04, 99.37, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_30GR_od19_84 = [ 1.08, 1.19, 1.28, 1.28, 1.28, 1.28, 1.28, 1.28, 1.28, 1.28, 1.37, 1.50, 1.70, 1.91, 2.14, 2.45, 2.89, 3.59, 4.96, 9.26, 21.13, 42.65, 69.46, 90.32, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_30GR_od16_64 = [ 1.34, 1.48, 1.59, 1.68, 1.78, 1.88, 1.97, 1.97, 1.97, 2.06, 2.16, 2.30, 2.52, 2.75, 2.98, 3.30, 3.73, 4.44, 5.85, 10.46, 23.76, 47.74, 76.14, 92.11, 97.37, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_30GR_od07_38 = [ 8.09, 9.59, 10.86, 11.92, 13.20, 14.50, 15.46, 6.35, 17.12, 17.71, 18.27, 18.98, 20.05, 21.20, 2.48, 24.29, 26.55, 29.80, 34.86, 45.30, 62.11, 80.83, 93.74, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_30GR_od22_13 = [ 1.32, 1.48, 1.60, 1.70, 1.82, 1.93, 2.02, 2.12, 2.12, 2.21, 2.32, 2.46, 2.68, 2.90, 3.14, 3.47, 3.93, 4.70, 6.09, 10.07, 20.37, 38.47, 60.39, 79.95, 92.87, 98.84, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_30GR_od14_21 = [ 1.99, 2.23, 2.41, 2.56, 2.74, 2.92, 3.06, 3.20, 3.34, 3.46, 3.59, 3.78, 4.06, 4.36, 4.65, 5.03, 5.49, 6.19, 7.54, 11.87, 24.41, 48.74, 80.55, 98.71, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_30GR_od09_15 = [ 3.94, 4.61, 5.17, 5.65, 6.22, 6.80, 7.23, 7.62, 7.97, 8.25, 8.53, 8.89, 9.44, 10.01, 10.60, 11.41, 12.38, 13.78, 16.37, 23.89, 41.22, 66.35, 88.63, 98.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00, 100.00 ] supertab_30GR_ave = [np.average(item) for item in zip(supertab_30GR_od05_40, supertab_30GR_od14_59, supertab_30GR_od14_16, supertab_30GR_od12_68, supertab_30GR_od19_84, supertab_30GR_od16_64, supertab_30GR_od07_38, supertab_30GR_od22_13, supertab_30GR_od14_21, supertab_30GR_od09_15) ] supertab_30GR_std = [np.std(item) for item in zip(supertab_30GR_od05_40, supertab_30GR_od14_59, supertab_30GR_od14_16, supertab_30GR_od12_68, supertab_30GR_od19_84, supertab_30GR_od16_64, supertab_30GR_od07_38, supertab_30GR_od22_13, supertab_30GR_od14_21, supertab_30GR_od09_15) ] supertab_30GR = [ supertab_30GR_od05_40, supertab_30GR_od14_59, supertab_30GR_od14_16, supertab_30GR_od12_68, supertab_30GR_od19_84, supertab_30GR_od16_64, supertab_30GR_od22_13, supertab_30GR_od14_21, supertab_30GR_od09_15 ] # Define the Sigmoidal function. def sigmoid(x, a, b, k, t): """ Sigmoidal function. x = Input data. a = Minimum value of input data. b = Maximum value of input data. k = Midpoint of the Sigmoid. t = Rate of increase. """ y = a + (b/(1 + np.exp((k - x)/t))) return y # Get the 10, 50, and 90th percentiles. pharmatose_150M_percentiles = [] for item in pharmatose_150M: popt, pcov = curve_fit(sigmoid, size, item) _10 = popt[2] - popt[3]*np.log(popt[1]/(10 - popt[0]) - 1) _50 = popt[2] - popt[3]*np.log(popt[1]/(50 - popt[0]) - 1) _90 = popt[2] - popt[3]*np.log(popt[1]/(90 - popt[0]) - 1) temp = _10, _50, _90 pharmatose_150M_percentiles.append(temp) supertab_11SD_percentiles = [] for item in supertab_11SD: popt, pcov = curve_fit(sigmoid, size, item) _10 = popt[2] - popt[3]*np.log(popt[1]/(10 - popt[0]) - 1) _50 = popt[2] - popt[3]*np.log(popt[1]/(50 - popt[0]) - 1) _90 = popt[2] - popt[3]*np.log(popt[1]/(90 - popt[0]) - 1) temp = _10, _50, _90 supertab_11SD_percentiles.append(temp) supertab_30GR_percentiles = [] for item in supertab_30GR: popt, pcov = curve_fit(sigmoid, size, item) _10 = popt[2] - popt[3]*np.log(popt[1]/(10 - popt[0]) - 1) _50 = popt[2] - popt[3]*np.log(popt[1]/(50 - popt[0]) - 1) _90 = popt[2] - popt[3]*np.log(popt[1]/(90 - popt[0]) - 1) temp = _10, _50, _90 supertab_30GR_percentiles.append(temp) # Create a list of the generated percentile values. pharmatose_150M_10 = [ 119.34, 121.96, 125.33, 129.43, 114.13, 120.18, 128.50, 107.36, 125.58, 127.73 ] pharmatose_150M_50 = [ 191.16, 178.32, 190.26, 196.19, 175.81, 195.76, 197.87, 171.37, 201.16, 208.96 ] pharmatose_150M_90 = [ 258.28, 236.00, 262.29, 281.83, 242.86, 289.82, 283.27, 241.46, 278.42, 308.22 ] pharmatose_150M_reported = [ pharmatose_150M_10, pharmatose_150M_50, pharmatose_150M_90 ] supertab_11SD_10 = [ 114.96, 118.13, 112.20, 127.81, 125.54, 129.74, 104.16, 118.42, 126.26 ] supertab_11SD_50 = [ 172.99, 176.09, 170.03, 194.41, 198.26, 208.57, 169.02, 192.88, 208.49 ] supertab_11SD_90 = [ 229.41, 233.45, 232.95, 271.49, 290.66, 336.04, 230.22, 292.81, 325.82 ] supertab_11SD_reported = [ supertab_11SD_10, supertab_11SD_50, supertab_11SD_90 ] supertab_30GR_10 = [ 140.35, 131.52, 129.25, 124.65, 126.56, 123.02, 124.67, 116.38, 44.88 ] # 5.82 supertab_30GR_50 = [ 207.76, 196.63, 196.89, 188.90, 189.60, 182.79, 131.99, 198.41, 181.39, 160.48 ] supertab_30GR_90 = [ 280.54, 271.11, 290.39, 272.56, 254.39, 249.72, 204.86, 293.91, 235.82, 220.86 ] supertab_30GR_reported = [ supertab_30GR_10, supertab_30GR_50, supertab_30GR_90 ] for i in range(0, 3): rept_ave = np.average(pharmatose_150M_reported[i]) calc_ave = np.average([item[i] for item in pharmatose_150M_percentiles]) rept_std = np.std(pharmatose_150M_reported[i]) calc_std = np.std([item[i] for item in pharmatose_150M_percentiles]) print 'Calculated average for Pharmatose 150M = %f' % calc_ave print 'Reported average for Pharmatose 150M = %f' % rept_ave print 'Calculated STD for Pharmatose 150M = %f' % calc_std print 'Reported STD for Pharmatose 150M = %f' % rept_std for i in range(0, 3): rept_ave = np.average(supertab_11SD_reported[i]) calc_ave = np.average([item[i] for item in supertab_11SD_percentiles]) rept_std = np.std(supertab_11SD_reported[i]) calc_std = np.std([item[i] for item in supertab_11SD_percentiles]) print 'Calculated average for SuperTab 11SD = %f' % calc_ave print 'Reported average for SuperTab 11SD = %f' % rept_ave print 'Calculated STD for SuperTab 11SD = %f' % calc_std print 'Reported STD for SuperTab 11SD = %f' % rept_std for i in range(0, 3): rept_ave = np.average(supertab_30GR_reported[i]) calc_ave = np.average([item[i] for item in supertab_30GR_percentiles]) rept_std = np.std(supertab_30GR_reported[i]) calc_std = np.std([item[i] for item in supertab_30GR_percentiles]) print 'Calculated average for SuperTab 30GR = %f' % calc_ave print 'Reported average for SuperTab 30GR = %f' % rept_ave print 'Calculated STD for SuperTab 30GR = %f' % calc_std print 'Reported STD for SuperTab 30GR = %f' % rept_std
sizing lactose 01
Posted by () in Open Notebook Science, powder_tapping on May 18, 2013
Particle sizing
I was able to size the lactose particles after sieving using the SympaTEC HELOS laser diffractor in the lab. I unfortunately had to do this step multiple times as I was having some difficulty interpreting the results. Nonetheless, I believe I understand how the device works now.
The sizer uses a laser to illuminate particles that are suspended in mineral oil that has 1% Span-85 in it. The laser is collimated and has a beam width of about 2 cm. A lens is then used to focus the light from the sample onto a detector. The detector is placed in the Fourier plane of the lens in order to detect diffraction patterns from the particles. Using Fraunhofer diffraction theory, a particle size is estimated from its diffraction pattern.
What caused my initial hesitation about the results was that I was told to not put a lot of particles in the cuvette—so I didn’t. However, I was told to add more particles to the cuvette and take successive measurements. I found documentation on the computer that states one should shoot for a 10–15% optical density which, at the time of my first run with the machine, I had no clue as to what that meant. My main cause of confusion and why I redid the sizing was when I added particles to the cuvette, it caused the sizing results to shift slightly.
My second go with the machine allowed for more clarity. I understand the caution for not putting a ton of particles in the cuvette since you don’t want to saturate the detector. If there are too many particles, than the detector (which I’m assuming is nothing more than a camera with multiple arrays) is unable to detect individual diffraction patterns through the software. I did oversaturate the detector once but, I just waited a couple of minutes till the particles settled in the cuvette. I then retook the measurement and sure enough, the detector wasn’t saturated. Of course doing this skews the particle size results as the detector is detecting particles small enough that stayed in solution. The sizer indicates the “optical density” of a measurement and I believe this is a measure of the turbidity of the solution in the cuvette. I was able to get it as high as 44% optical density without the software complaining.
The data I obtained shifted dependent upon the time in which particles were allowed to be in the cuvette—i.e. larger ones sank. So, I made sure to load the cuvette with tons of particles, wait till it was able to detect them, and then took measurements as particles sunk. I also added particles to the cuvette over time adding small amounts as was instructed for me to do. Doing this gave a spread in sizing that I’m assuming comes from a spread in particles after sieving.
The SympaTEC software gives a plot of sizing data and shows the 10th, 50th, and 90th percentile of the sizes. I believe it is doing this by fitting a Sigmoid to the size data and finding on the curve the corresponding percentiles. This data is of course plotted in a proprietary .bmp format that is completely useless. So, I’m working on a script to plot the data which, thankfully was output in a txt file. For all intents and purposes I believe using the data that was output by the device is sufficient for sizing purposes. I’m just wanting to have a little fun with the data it gave me.
sieving analysis
Posted by () in Open Notebook Science, powder_tapping on May 18, 2013
I finished my sieving analysis and writeup for it. Below is the python script that I used to plot the data shown in the graphs below. I don’t know why but wordpress is removing my white space formatting.
# Import statements.
import matplotlib.pyplot as plt
from matplotlib import rcParams
# Change the figure size and font used.
rcParams['font.sans-serif'] = 'Arial'
rcParams['figure.figsize'] = [3.27, 3.27]
rcParams['xtick.direction'] = 'out'
rcParams['ytick.direction'] = 'out'
# Input data. Values are in grams.
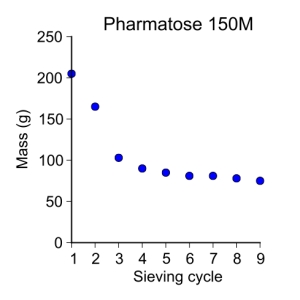
pharmatose150M = [ 205, 165, 103, 90, 85, 81, 81, 78, 75 ]
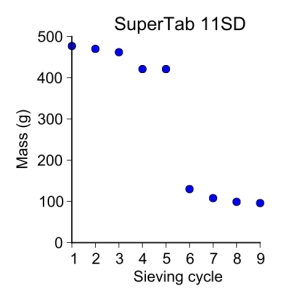
supertab11SD = [ 477, 470, 462, 421, 421, 130, 108, 99, 96 ]
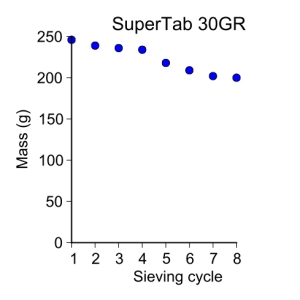
supertab30GR = [ 246, 239, 236, 234, 218, 209, 202, 200 ]
# Generate the x-axis.
sieve_cycle = [ 1, 2, 3, 4, 5, 6, 7, 8, 9 ]
# Plot the data.
def plot_data(x, y, marker_type, marker_size, x_label, y_label, title):
""" Plots the input data.
x = x-data
y = y-data
marker_type = Marker type of the plot.
marker_size = Marker size of the plot.
x_label = x-axis label.
y_label = y-axis label.
title = Title of the plot.
"""
# Initialize a figure.
fig = plt.figure()
ax = fig.add_subplot(1, 1, 1)
# Add labels.
ax.set_xlabel(x_label)
ax.set_ylabel(y_label)
ax.set_title(title)
# Fix the x-axis limit.
ax.set_xlim([1, 10])
# Modify the spines so that the top and right spines are not visible.
ax.xaxis.set_ticks_position('bottom')
ax.yaxis.set_ticks_position('left')
ax.spines['right'].set_visible(False)
ax.spines['top'].set_visible(False)
ax.spines['left'].set_smart_bounds(True)
ax.spines['bottom'].set_smart_bounds(True)
#ax.spines['left'].set_position(('axes', -0.01))
# Plot the data.
data = ax.plot(x, y, marker_type, marker_size)
for artist in data:
artist.set_clip_on(False)
fig.tight_layout()
fig.savefig(title.lower().replace(' ', '') + '.pdf')
plot_data(sieve_cycle, pharmatose150M, 'o', 3, 'Sieve cycle', 'Mass (g)',
'Pharmatose 150M')
plot_data(sieve_cycle, supertab11SD, 'o', 3, 'Sieve cycle', 'Mass (g)',
'SuperTab 11SD')
plot_data(sieve_cycle[:-1], supertab30GR, 'o', 3, 'Sieve cycle', 'Mass (g)',
'SuperTab 30GR')
sieving lactose 03
Posted by () in Open Notebook Science, powder_tapping on May 5, 2013
Last time I did a bulk sieving of the powders in order to fractionate them into different distributions of grain sizes. From this initial sieving, I saw that the largest amount of powder in each of the sieved sizes was the 125–150 µm range. If the Pharmatose 150M lactose took approximately the same number of sieving cycles as the SuperTabs than I wouldn’t be doing this extra sieving step, however, because it took 8 cycles I am not convinced that the sieving is complete enough to give large cutoffs for the 125–150 µm size range. This is why I have decided to sieve each of the 125–150 µm sizes again to ensure that sieving is producing a strong cutoff between sizes. Like before, I’ve set up the following sieves:
- Lid
- 250 µm
- 125 µm
- 75 µm
- Bucket
this time ignoring the 45 µm sieve since it has a hole in it. I put the 125–150 µm size range in the top sieve and let it shake for 30 minutes. I’m only interested in the 125–250 µm range so this is the only one that I will be measuring.
SuperTab 30GR
Initial conditions:
- 2014-05-04
- Temperature = 22.9˚C
- Relative humidity = 22%
My first sieving for the second sieve cycle was:
- 125–250 µm = 218 g
I’ll note that the powder seemed to be have somewhat of a charge to it and as such, seemed to cling to the sieve. As seen in a previous post, this weight is significantly different than the one I ended with so, time for another sieve cycle.
My second sieving for the second sieve cycle was:
- 125–250 µm = 209 g
My third sieving for the second sieve cycle was:
- 125–250 µm = 202 g
My fourth sieving for the second sieve cycle was:
- 125–250 µm = 200 g
Pharmatose 150M
My first sieving for the second sieve cycle was:
- 125–250 µm = 75 g
Since the last sieving of the Pharmatose 150M was at 78 g, this change is within the 1–5% weight change and thus I will not be sieving it more.
SuperTab 11SD
I separated the SuperTab 125–250 µm fraction because it was quite large. So I started off with about 150 g of it in the sievers.
My first sieving for the second sieve cycle was:
- 125–250 µm = 130 g
My second sieving for the second sieve cycle was:
- 125–250 µm = 108 g
My third sieving for the second sieve cycle was:
- 125–250 µm = 99 g
My fourth sieving for the second sieve cycle was:
- 125–250 µm = 96 g
The powders are now within the arbitrarily decided 1–5% change of weight between sieving cycles. The next step is to size the particles to see if they actually are within these ranges.
sieving lactose 02
Posted by () in Open Notebook Science, powder_tapping on April 30, 2013
Continuing from yesterday, I am sieving the SuperTab 11SD lactose. Except today I have split the large fraction between 125–250 µm into two batches. I will sieve both batches for 30 minutes.
- 23.1˚C
- 47% relative humidity
SuperTab 11SD
My fourth sieving obtained the following:
- > 250 µm = 8 g
- 125–250 µm = 421 g
- 75–125 µm = 14 g
- 45–75 µm = < 1 g
- < 45 µm = 37 g
My fifth sieving obtained the following:
- > 250 µm = 7 g
- 125–250 µm = 421 g
- 75–125 µm = 13 g
- 45–75 µm = < 1 g
- < 45 µm = 39 g
This looks like a good stopping point as I’m wanting to use the 125–250 µm fraction and it hasn’t change appreciably.
Pharmatose 150M
This powder is much more cohesive than the SuperTab powders. I’ll also note that the bag of lactose, when received, was open in the jar. This is fine as the powder was exposed to the elements during shipping and thus may have more moisture content in it than the other powders. A comparison can now be made between powders and their moisture content—dependent on if the moisture content is greater in the pharmatose powder.
My first sieving obtained the following:
- > 250 µm = 9 g
- 125–250 µm = 205 g
- 75–125 µm = 191 g
- 45–75 µm = 21 g
- < 45 µm = 68 g
My second sieving obtained the following:
- > 250 µm = 5 g
- 125–250 µm = 165 g
- 75–125 µm = 209 g
- 45–75 µm = 4 g
- < 45 µm = 109 g
My third sieving obtained the following:
- > 250 µm = 5 g
- 125–250 µm = 103 g
- 75–125 µm = 223 g
- 45–75 µm = 4 g
- < 45 µm = 153 g
Sieving the Pharmatose 150M is _very_ different than sieving the SuperTab powders. There seems to be a large amount of grains that are less than 45 µm in the batch and are for some reason getting stuck in the various sieves and powders in the sieves probably due to cohesiveness. Not sure though. I am making sure that the sieves are clear between each cycle. I suspect that more sieving sessions will refine the particle distributions but, I may end up with a bunch of powder with particle sizes less than 45 µm. I hope that the particles in the 125–250 µm range are for the most part separated nicely as this is the fraction that I wish to use in further experiments.
My fourth sieving obtained the following:
- > 250 µm = 5 g
- 125–250 µm = 90 g
- 75–125 µm = 201 g
- 45–75 µm = 14 g
- < 45 µm = 175 g
As suspected, I lost more in the 125–250 µm range. There still seems to be enough for this project but I need to go through another cycle since there was a 10% change in weight between sieving 3 and 4. I’ll also note that the powder in the 125-250 µm range is flowing better out of the sieve and into the jar I am putting it in. I’m guessing because the number of smaller particles in this fraction are becoming less.
My fifth sieving obtained the following:
- > 250 µm = 5 g
- 125–250 µm = 85 g
- 75–125 µm = 171 g
- 45–75 µm = 2 g
- < 45 µm = 207 g
My sixth sieving obtained the following:
- > 250 µm = 5 g
- 125–250 µm = 81 g
- 75–125 µm = 144 g
- 45–75 µm = 2 g
- < 45 µm = 235 g
I will do one more since the diference between the 75–125 is still above a 10% change in weight. I do know why my 45–75 µm fraction is always small, it’s because there is a rip in the sieve where it meets the holder. No wonder why I was not getting any in that fraction for all the powders. This isn’t a problem, it just means that my smallest fraction is actually < 75 µm and not < 45 µm. I’ll fix my notes after the seventh sieving just so I stay consistent with what I started, i.e. so I don’t mess up with this final sieve.
My seventh sieving obtained the following:
- > 250 µm = 5 g
- 125–250 µm = 81 g
- 75–125 µm = 144 g
- 45–75 µm = 2 g
- < 45 µm = 235 g
My eighth sieving obtained the following:
- > 250 µm = 4 g
- 125–250 µm = 78 g
- 75–125 µm = 119 g
- 45–75 µm = X g
- < 45 µm = 262 g
I have to admit that this was fairly tedious and left me guessing if the sieving actually worked. I am wanting to ensure that the distribution in sizes of the particles are what I think they are so I may end up sieving the 125–250 µm fraction for all lactose types again and ensuring that the weight change between sieving cycles is less than 5%. I want to use the 125–250 µm particle size range as all three powders have > 50 g of powder in that range. So, I think I will end up sieving this range again just to make sure that the particle distribution is as narrow as I think it should be. Of course I will need to size the particles after the sieving process is complete.
I will also note that the Pharmatose 150M lactose in the 125–250 µm size range was pretty cohesive when I was trying to empty the powder out of the sieves for the first couple of cycles. After about the 5th one, the particles started flowing out much more smoothly and I think this is because the smaller particles were leaving this fraction. Still, I think I need to sieve the particles some more next time I’m in.
sieving lactose
Posted by () in Open Notebook Science, powder_tapping on April 29, 2013
Introduction
This project is a continuation of initial experiments done by Sarah, Damian and Jihyun. The experiments that I will conduct are basic refinements from their initial experiments and will serve as a metric for more standardized tests that can be done with powders. The goal will be to investigate the gold standards for powder characterization for pharmaceutical excipients and compare them to our novel tapping apparatus.
Sieving
I received lactose powder from DFEPharma on 2013-04-25 and am in the process of sieving it. I received three types of powders:
where GR=granulated, SD=spray dried, and M=milled lactose types. My process for sieving consists of the following steps:
- Weigh the initial amount of powder received.
- Stack the following sieves from top (1) to bottom (6):
- Lid
- 250 µm sieve
- 125 µm sieve
- 75 µm sieve
- 45 µm sieve
- Collection bucket.
- Place all the powder in the top most sieve and let the sieving machine go for 30 minutes—this is the first sieving. The machine is a Tyler RX-24 Portable Sieve Shaker.
- Take each fraction and weigh the amount of powder in each sieve.
- Return the powder to its respective sieve and allow the machine to shake for 30 more minutes.
- Repeat until there is no appreciable weight change between sieves for each fraction.
The sieving machine can be seen in action in the below video. It’s a loud machine but, very effective at what it does.
SuperTab 30GR
I started with the SuperTab 30GR granulated lactose powder. The ambient climate here in Austin for 2013-04-29 and my initial powder weight conditions are:
- Temperature = 23.5˚C
- Relative humidity = 47%
- Initial amount of powder = 498 g
- Powder name: Monohydrate Lactose USP/NF, Ph. Eur., JP
- Product code: 42320-6460
- Product date: 04-2012
- Charge Number: 10637764
- ME/SU number: 5633687
- Expiration date: 03-2015
- Drum number: 854
I had some difficulty starting the machine due to not tightening the clamps that hold the sieve down enough, however, things are moving smoothly now. For my first sieve, I obtained the following fractions:
- > 250 µm = 42 g
- 125–250 µm = 246 g
- 75–125 µm = 122 g
- 45–75 µm < 1 g
- < 45 µm = 84 g
where the weight measurements are ±1 g. My second sieve obtained the following fractions:
- > 250 µm = 40 g
- 125–250 µm = 239 g
- 75–125 µm = 126 g
- 45–75 µm < 1 g
- < 45 µm = 88 g
My third sieving obtained the following:
- > 250 µm = 39 g
- 125–250 µm = 236 g
- 75–125 µm = 127 g
- 45–75 µm < 1 g
- < 45 µm = 90 g
My fourth sieving obtained the following:
- > 250 µm = 38 g
- 125–250 µm = 234 g
- 75–125 µm = 129 g
- 45–75 µm < 1 g
- < 45 µm = 91 g
It would appear that I’ve reached enough of a plateau to stop. The percent change between sieves is between 1–5% and I’m okay with this. On to the next powder.
SuperTab 11SD
My initial conditions are:
- Temperature = 23.5˚C
- Relative humidity = 47%
- Initial amount of powder = 513 g
- Powder name: Monohydrate Lactose USP/NF, Ph. Eur., JP
- Product code: 42332-6456
- Product date: 11-2012
- Charge Number: 10678880
- ME/SU number: 5727977
- Expiration date: 10-2014
- Drum number: 976
My first sieving obtained the following:
- > 250 µm = 9 g
- 125–250 µm = 477 g
- 75–125 µm = 4 g
- 45–75 µm = 1 g
- < 45 µm = 17 g
This powder is much more uniform in its size distribution than the granulated lactose. It also seems to be more cohesive which, is a completely qualitative statement right now.
My second sieving obtained the following:
- > 250 µm = 8 g
- 125–250 µm = 470 g
- 75–125 µm = 5 g
- 45–75 µm = < 1 g
- < 45 µm = 24 g
My third sieving obtained the following:
- > 250 µm = 6 g
- 125–250 µm = 462 g
- 75–125 µm = 6 g
- 45–75 µm = < 1 g
- < 45 µm = 28 g
I think I may have to break up the 125–250 µm fraction. It looks like the siever may be overloaded and would benefit from only having half as much powder in the 125–250 sieve for a cycle.